

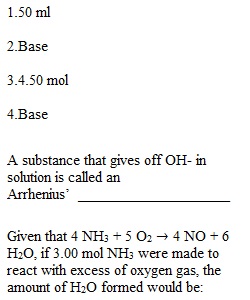
Q Question 1 3 / 3 pts How many mL of a 2.0M NaBr solution are needed to make 200.0 mL of 0.50M NaBr? Question 2 1 / 1 pts A substance that accepts H+ in solution is called a Bronsted-Lowry’s _______________ Question 3 3 / 3 pts Given that 4 NH3 + 5 O2 ? 4 NO + 6 H2O, if 3.00 mol NH3 were made to react with excess of oxygen gas, the amount of H2O formed would be: Question 4 1 / 1 pts A substance that gives off OH- in solution is called an Arrhenius’ ___________________
View Related Questions