

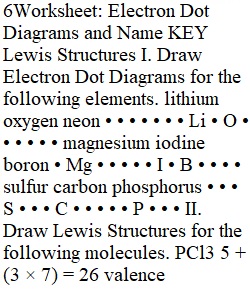
Q 6Worksheet: Electron Dot Diagrams and Name KEY Lewis Structures I. Draw Electron Dot Diagrams for the following elements. lithium oxygen neon • • • • • • • Li • O • • • • • • magnesium iodine boron • Mg • • • • • I • B • • • • sulfur carbon phosphorus • • • S • • • C • • • • • P • • • II. Draw Lewis Structures for the following molecules. PCl3 5 + (3 × 7) = 26 valence e- CH4 4 + (4 × 1) = 8 valence e- H • • • Cl • • • • • • ? P ? Cl • | • • | H ? C ? H • Cl • | • • • • H CH3Br 4 + (3 × 1) + 7 = 14 valence e- H F2O 6 + (2 × 7) = 20 valence e- | H ? C ? H | • Br • • • • • • ? O • • • | • • • • • • • • • • IBr 7 + 7 = 14 valence e- NH2Cl 5 + (2 × 1) + 7 = 14 valence e- • • • • • ? Br • • • • • • • H ? N ? H | • Cl • • • • • CHEMISTRY: A Study of Matter © 2004, GPB 5.11a Worksheet on Lewis Structures 3 LewisStructuresHwrk.odt Solutions to Lewis Structures Homework 1. PI3 2. N2 3. H2O 4. AsBr3 5. SiCl4
View Related Questions